Superbugs: why it’s so hard to stop the ‘silent pandemic’ But there is little incentive for Big Pharma to tackle the issue
In the summer of 2021, researchers at MIT and McMaster University in Canada fed an algorithm 7,000 chemical compounds in the hope that it would identify one that could kill Acinetobacter baumannii.
Described by Jonathan Stokes, one of the scientists involved, as a “notoriously challenging” pathogen, strains of Acinetobacter have become resistant to antibiotics over the past few decades, allowing them to prey on weakened hospital patients and leaving doctors powerless to treat them.
It took just an hour and a half — a long lunch — for the AI to serve up a potential new antibiotic, an offering to a world contending with the rise of so-called superbugs: bacteria, viruses, fungi and parasites that have mutated and no longer respond to the drugs we have available.
After the AI identified the compound, the researchers refined it to make it more powerful. Then they tested it in mice, finding it could suppress the bacteria in wound infections. (Compared to traditional methods, the algorithm is better at finding compounds that work in animals; it has already found several other candidates.) It will take years to test the drug in humans and find out if the AI really has hit gold. But Stokes is enthusiastic. “I’m really excited about this compound. I love this compound,” he says.
Antimicrobial resistance (AMR) — which encompasses all microbes and not just bacteria, which are targeted by antibiotics — is sometimes referred to as a “silent pandemic”. Resistant pathogens killed 1.26mn people in 2019, according to an analysis published in the medical journal The Lancet. “All of modern medicine is upheld by our ability to control infectious disease. If we can’t control infection, we can’t administer chemotherapy, do invasive surgery, and preterm birth becomes really, really challenging and risky,” Stokes says.
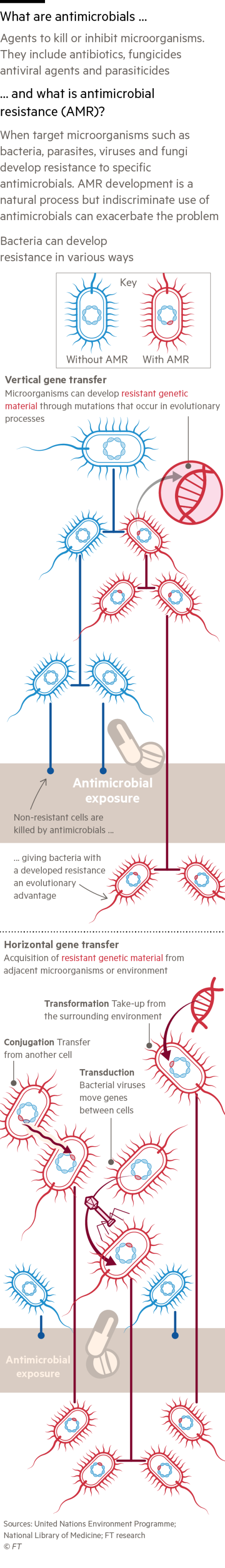
The problem is getting worse with time. In 2016, a UK review led by Lord Jim O’Neill, an economist and former Goldman Sachs banker, forecast the number of annual deaths from antimicrobial resistance would rise to 10mn by 2050 — approximately the number of people who currently die from cancer. But based on more recent data, he now believes that up to twice as many could die.
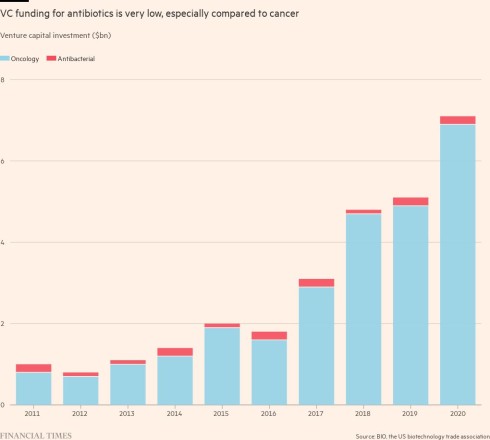
The pharmaceutical industry and governments are failing to invest enough in replacing the older antibiotics with newer drugs that bacteria aren’t resistant to, risking crises where clinicians — whether they are treating one patient or a pandemic — find the medicine cabinet is in effect bare.
Technologies such as AI could help combat resistance by cutting the time and cost of the initial phase of drug discovery, while portable genomic sequencing technology could help doctors choose the right antibiotic for each pathogen in the clinic or hospital.
But even when a promising new antibiotic is discovered, it enters a broken market. To avoid spurring yet more resistance, new antibiotics should be used sparingly, so they are unlikely to be bestsellers for drug companies. Governments and health systems accustomed to cheap generic antibiotics will not spend enough on novel drugs to make antibiotic development pay off. The cost of bringing a new antibiotic to market is approximately $1.5bn.
Few venture capitalists or large drugmakers want to fund the costly clinical trials required by regulators. Investors have lost about $4bn on biotechs developing antibiotics, according to the impact investor the AMR Action Fund. The start-ups have either gone bankrupt, been sold off cheap, or pivoted to more lucrative areas.
As with climate change and future pandemics, no one is taking enough responsibility for the ever-present global threat of antimicrobial resistance, scientists say. The UK, US and EU are working on ways to incentivise drugmakers to create better antibiotics, but so far, their efforts have lacked co-ordination and urgency.
O’Neill says the Covid-19 pandemic demonstrated how “devastating” uncontrolled infectious disease can be. But as people try to return to normality, he says it has become a “tough sell” for policymakers to “bombard people with dreadful views of the future all the time”.
“Unless it gets on the 10 o’clock news, one of the top stories each day of the week, which policymakers are really going to put it right at the top of the agenda, including putting money behind it as needed? And the answer is, I can’t think of any,” he says.
Overuse and resistance
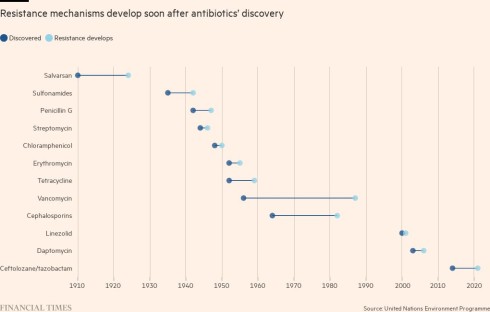
When British scientist Sir Alexander Fleming gave his acceptance speech for winning the 1945 Nobel Prize for discovering penicillin, he warned of the dangers of rising resistance. “The time may come when penicillin can be bought by anyone in the shops. Then there is the danger that the ignorant man may easily underdose himself and by exposing his microbes to non-lethal quantities of the drug make them resistant,” he said.
Fleming had foresight. Overuse of antibiotics is a large contributor to antimicrobial resistance, particularly in the developing world where the drugs are often available without a prescription, and in the US, where doctors frequently prescribe them for infections that may not be caused by a bacteria, such as a cold. Even in the UK, where the NHS is more cautious about doling out prescriptions, the former health secretary Thérèse Coffey admitted handing leftover antibiotics to her friends.
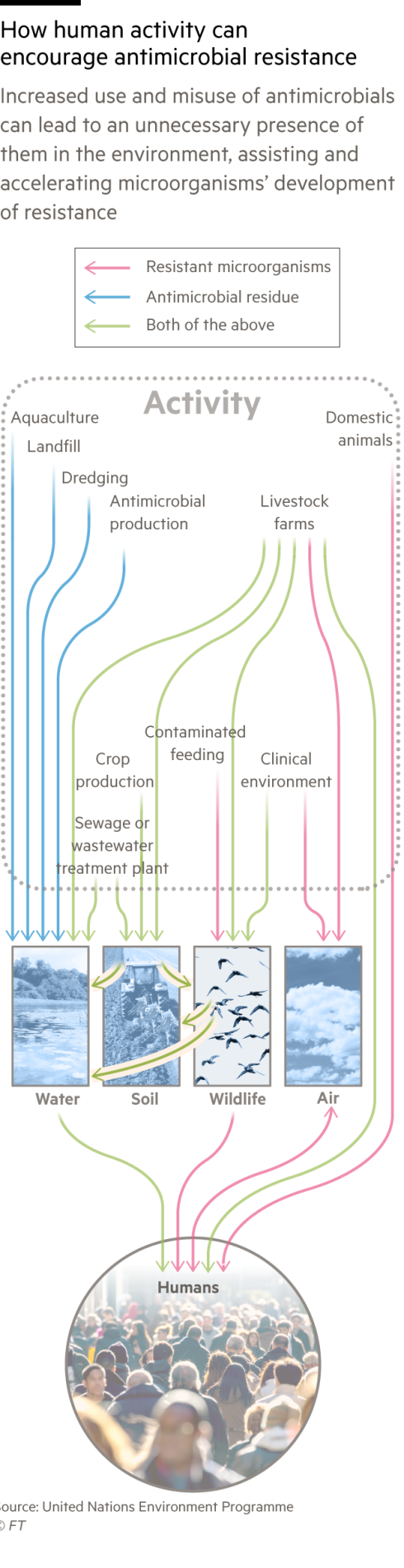
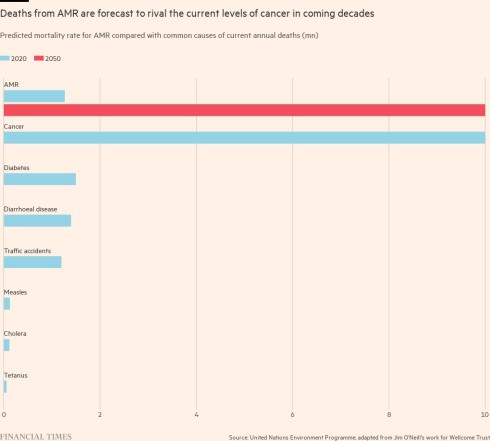
The more bacteria are exposed to antibiotics, the more they evolve ways to avoid their killing mechanisms and survive. As well as overprescription in humans, bacteria are exposed to antibiotics in the food supply chain, where animals are pumped with the drugs to avoid disease in cramped conditions and, in some cases, to boost their growth. Other factors are emerging: a separate recent study in The Lancet also suggested that air pollution may be a vector for superbugs, as resistance rises in tandem with levels of small particulate matter.
But even clinicians who are aware of the problem, and want to give antibiotics in a more targeted way, struggle because of a lack of diagnostics — tests that can identify precisely what the pathogen is. They tend to rely on so-called broad spectrum antibiotics, which should be able to tackle a range of bacteria, but have the serious side effect of building resistance even in bacteria they are not targeting.
At London’s St Thomas’s hospital, on the banks of the river Thames, microbiologists are trialing a new approach to speed up diagnosis and alert doctors to when their patient has a bacteria that may be resistant to an antibiotic.
Instead of waiting three to five days for scientists to grow the bacteria in a Petri dish and examine it under a microscope, they are using a genomic sequencer developed by Oxford Nanopore. The size of a printer, it can give its first view on what the pathogen is within half an hour, and a full report in two hours.
Previously the reports from the microscope observations had been “close to being completely unhelpful”, says Jonathan Edgeworth, a consultant microbiologist at St Thomas’s and the vice-president of medical affairs at Nanopore. They were viewed by doctors as a public health tool to track disease, not diagnose it. Now, they can use the sequencer to select the right treatment, and eventually, the data could also point drugmakers to which resistant pathogens to target and how.
Ian Abbs, chief executive of the hospital’s trust, says they have already seen an impact in intensive care patients, where the potential financial savings of treating patients more quickly are significant. “Each day costs about £2,500, depending on the complexity of the patient. For my sickest patients, it could be £10,000,” he says. The scheme is being expanded to five other hospitals and Abbs hopes to quickly spread it across the NHS.
Incentivising research
To keep research labs open and looking for new antibiotics, philanthropists and impact investors have tried to fill the gap that venture capitalists have left.
In 2016, a US-based consortium called CARB-X launched with government and foundation money to accelerate development of new antibiotics, vaccines and rapid diagnostics. That year, the World Health Organization and the Drugs for Neglected Diseases Initiative created GARDP, a partnership to accelerate the development of treatments for drug-resistant infections. In 2020, drugmakers invested about $1bn in the AMR Action Fund, aiming to launch two to four new antimicrobials in the next decade.
The researchers at McMaster and MIT have given their potential drug to Phare Bio, a social venture that has raised $25mn from The Audacious Project, which combines funding from TED (of TED Talks fame) and other non-profits. It is testing the drug candidate in animal studies and hopes to partner with Big Pharma to get it through clinical trials.
“Because we have philanthropic investment to help us get through this highest risk phase, we feel that will enable us to succeed and in ways that companies that are only commercially funded even at the earliest stages may not be able to,” Akhila Kosaraju, a doctor who is now Phare Bio’s chief executive, explains.
But for all the optimism, Henry Skinner, chief executive of the AMR Action Fund, says AI is “helpful, certainly, but not transformative” because it does little to address where the real costs lie: in clinical trials. He thinks even his fund is “at best, a stop gap, partial solution”.
“We feel a huge amount of responsibility. One billion dollars sounds like a lot of money but it is not nearly enough for the very expensive last-stage work,” he says.
To create better incentives, attention is turning to changing how health systems buy antibiotics. This year, the UK has proposed expanding its novel subscription model, so drugmakers would receive up to £20mn a year for selling innovative antibiotics, no matter how many — or how few — are prescribed.
The pilot started with drugs developed by Pfizer and Japan’s Shionogi last year. Mark Hill, Shionogi’s global head of market access, believes it is a “very promising model” that encourages more investment, because you can prove to shareholders that you will get a return. “Unless you can get governments to think about this in a more creative way than the traditional supply, pay on demand per unit model, you can really struggle with your cash flow,” he says.
Patrick Holmes, global innovation policy lead at Pfizer, praised the UK for trying to value new antibiotics partly based on how they would affect resistance rates in the future.
The EU is planning to give drugmakers who bring a new antibiotic to market a voucher that can be used to extend the years of market exclusivity on another, presumably more profitable, drug, which it estimates will be worth about €440mn. Large drugmakers could use this for one of their own drugs, while smaller companies could sell the transferable voucher on.
But much is riding on whether the US, the world’s largest pharmaceutical market, can push through its Pasteur Act, which would also establish a subscription-style model, with contracts valued between $750mn and $3bn. Holmes says it is the only incentive large enough to drive a significant change in where drugmakers spend on research and development.
The act’s passage has not been smooth. It was originally introduced in 2020 and the budget has already been cut, from $11bn to $6bn. But after it was reintroduced in April this year, Mark McClellan, director of the Duke-Margolis Centre for Health Policy, is hopeful that the bipartisan bill could be tacked on to a bill on defence spending in the second half of this year.
“We’re aiming for a multibillion-dollar programme here, which might seem like a lot, but it’s actually low compared to the current and projected costs of not having antibiotics around that can treat the most important resistant organisms that are around today,” he says.
Yet even if western countries do find ways to fix their antibiotics markets, companies will still not be incentivised to launch novel antibiotics in developing countries. The problems of overreliance on broad spectrum antibiotics and a lack of diagnosis are likely to persist in regions without state of the art healthcare, and the resulting resistant superbugs are unlikely to respect national borders.
Jayasree Iyer, chief executive of the Access to Medicines Foundation, says she wants to see global action that will help the countries that struggle with the highest need and the biggest drug resistance problems. She says antimicrobial resistance is a global problem that you cannot tackle country by country, arguing that an incentive like the UK’s subscription model is not significant enough for drugmakers to then prioritise India, Thailand or South Africa.
“Everybody’s problem becomes nobody’s problem,” she says. “This has been on the political agenda for years now. Very little has been on the political agenda for this long.”
The UK recently announced an investment of £210mn in labs, technologies and people to track resistant pathogens across Asia and Africa. But neither western governments nor companies are significantly investing in making new antibiotics available in these countries.
An antimicrobial resistance expert at the World Health Organization says the agency is trying to promote a global approach. He warns that accessibility is becoming a problem, just like it was in the Covid-19 pandemic, when vaccine makers prioritised high-income countries, leaving developing countries behind.
O’Neill, the author of the UK review, cautiously welcomes the progress that has been made in the field in the past six months, with “some slight amazement” after years of little momentum. He believes the expansion of the UK subscription scheme, the EU proposal, and especially the size of the potential Pasteur Act, could encourage venture capitalists to support early research again.
But he called for more urgency. “It’s not like policymakers can sit around, trotting out these ideas and never following through,” he says. Otherwise, he warns, antimicrobial resistance may cause a crisis that will make Covid-19 look like a “garden party”.
Graphic illustrations by Ian Bott
This story originally appeared on: Financial Times - Author:Ian Bott
























