Blue blood of horseshoe crabs puts pharma groups under scrutiny
Tapping the blue blood of horseshoe crabs to test vaccines and medical devices is coming under fire, with environmentalists and a large European investor putting pressure on pharmaceuticals groups to seek alternatives.
The industry uses the blood in millions of tests a year to detect endotoxins, poisons inside bacteria cells that can make people ill. But pressure is mounting on pharma companies to switch to synthetic substances as populations decline for horseshoe crabs and seabirds that eat the creatures’ eggs.
The asset management division of BNP Paribas, the French banking group, has sent letters to 14 of the world’s largest pharmaceutical companies asking them to use a cloned substance called recombinant factor C (rFC) instead of blood from horseshoe crabs for tests.
“Every injectable drug, including vaccines, every device that’s implanted in the body, including pacemakers, all of them depend on endotoxin testing, all of them depend on this one animal which is declining,” said Adam Kanzer, head of Americas stewardship at BNP Paribas Asset Management, which oversees €526bn.
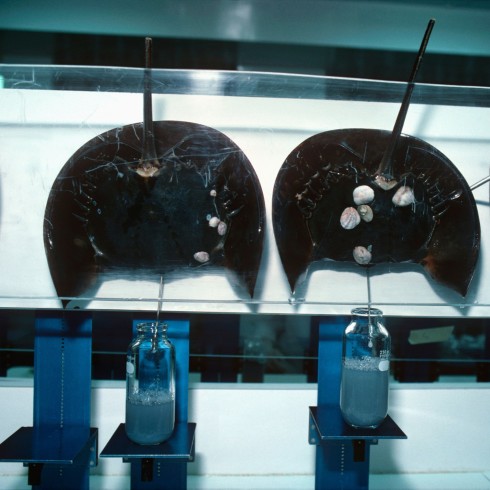
Horseshoe crabs, more closely related to spiders than crabs, have lived in the oceans for about 450mn years, before dinosaurs roamed the planet. But their numbers are falling because of habitat destruction and increased collection by the fishing and biomedical industries.
In 2019 one of Asia’s three species, the tri-spine horseshoe crab, was listed as endangered by the International Union for Conservation of Nature. The global environmental protection organisation lists the American horseshoe crab as “vulnerable”.
In the US, regulations allow the biomedical industry to collect a portion of a horseshoe crab’s blood and then release it alive in the area where it was collected. Almost 720,000 horseshoe crabs were harvested for blood extraction in 2021, according to US authorities, of which it is estimated that 112,104 died.
Horseshoe crabs have been a priority for environmentalists. Earthjustice, an environmental law group, named it as lobbying issue last year, according to US Senate disclosure filings. But before BNP got involved, protecting them had not been on the list of issues for environmental, social and governance (ESG) investors.
In May, the Pharmaceutical Supply Chain Initiative (PSCI), a lobby set up by large pharmaceutical companies, said its members should stop sourcing blood from the two Asian horseshoe crab species most at risk. The London-based PSCI said continuing to harvest horseshoe crabs for their blood “[may] lead to population decline”, and encouraged member companies “to explore and adopt alternatives”.
At Indianapolis-based Eli Lilly, about 80 per cent of endotoxin testing capacity has converted to rFC and regulators have approved eight products utilising these tests. The drugmakers Pfizer and Roche said they are piloting use of rFC at some of their facilities.
Jay Bolden, a biologist working at Lilly, said the company began to make the shift to rFC over concerns about possible supply chain “pinches” when US authorities designated the red knot, a seabird, as endangered in 2015. Red knots feed on horseshoe crab eggs as they migrate from Argentina to the Arctic.
“There are regulatory hurdles that make it difficult to completely change. But we are working on that,” said Bolden, who is a keen bird watcher.
One reason why synthetic tests have not been more widely adopted is a lack of standards on the use of rFC, according to Lonza, the Swiss-based biosciences company which has developed an alternative test. US Pharmacopeia (USP), a non-profit organisation that sets standards for medicines made in the US, has not published rFC standards, Lonza said.
Last year USP dismissed all members of a committee working on the rFC standards, citing an inability to work “collaboratively and productively, impeding the standards-setting process”.
USP said agreeing standards would be a “top priority” for a newly appointed committee, which includes Lilly’s Bolden as a member and is expected to announce an update as early as Tuesday.
“The pharmaceutical industry is conservative by nature . . . and it has taken industry a long time to wrap their heads around this,” Bolden said. “But it’s happened especially within the last year or two.”
This story originally appeared on: Financial Times - Author:Jamie Smyth



























