Weight-loss drugs: will health systems and insurers pay for ‘skinny jabs’?
When Jimmy Kimmel hosted the Academy Awards this year, the comedian opened by telling the audience how great they looked. Then he stung: “When I look around this room, I can’t help but wonder, ‘Is Ozempic right for me?’”
Kimmel was needling Hollywood for its newfound dependence on the diabetes medication that spurs weight loss, which has reportedly become de rigueur among the wealthy and famous.
Novo Nordisk, the company behind semaglutide — branded as Ozempic for diabetes and Wegovy for weight loss — has benefited from a surge in demand from patients inspired by celebrities’ transformations. But the drug’s reputation as a “skinny jab” has not helped persuade health systems and insurers, or payers, to cover the cost of it.
This week, the Danish drugmaker published headline trial data that it believes will make the difference. In a trial of patients with obesity and cardiac conditions, Wegovy was shown to cut the risk of serious events such as heart attacks and strokes by 20 per cent, proving that the drug improves heart health — and has the potential to reduce high healthcare costs.
Lars Fruergaard Jørgensen, Novo Nordisk’s chief executive, tells the Financial Times that the “whole reason” the company did the trial was to help win the argument with payers.
Shares in the company and its main rival, the US pharma group Eli Lilly, shot to record highs, as investors hoped that payers would not be able to refuse to cover the drugs for much longer. Evan Seigerman, an analyst at the Canadian investment bank BMO, added tens of billions of dollars to his sales estimates for the weight loss and diabetes drugs after the announcement. He now forecasts the entire market will one day be worth $130bn to $140bn.
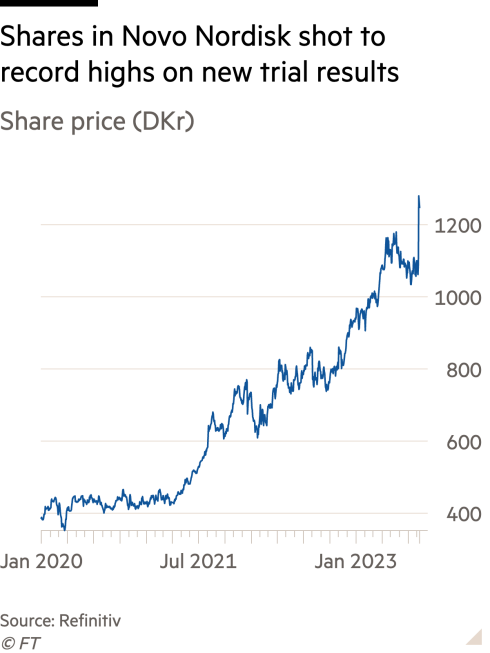
Drugmakers believe the medicines will help save on the healthcare costs related to obesity, which add up to about $170bn a year in the US, according to the Centers for Disease Control and Prevention.
However, health insurers and public payers argue that they have no way to magic up the money to pay for the drugs that would be needed to cater to a potential market of tens of millions of patients in the US alone, at a list price of about $1,300 a month, for the rest of their lives.
James Gelfand, president of the ERISA Industry Committee, which represents large US employers that provide health benefits, says the conflict is taking over his LinkedIn feed.
“It’s a war zone between patient advocates and doctors who are extolling the virtues of these drugs, and then plan sponsors, administrators, actuaries, etc, who are saying these drugs are a massive profiteering scheme,” he says.
Health effects
After decades of drugs that either offered lacklustre weight loss, or serious side effects, semaglutide was a breakthrough for the treatment of obesity.
First developed to treat diabetes, the compound was trialled by Novo in patients with obesity who were not diabetics. Results published in 2021 showed participants taking the weekly injection lost an average of about 15 per cent of their body weight, compared to about 2 per cent with just diet and exercise.
The next year, a trial showed that Eli Lilly’s similar drug tirzepatide could help patients on the highest dose lose an average of 22.5 per cent of their body weight. Tirzepatide is not yet approved for obesity, though the same active ingredient is available branded as Mounjaro, for diabetes.
The drugs have side effects — most commonly nausea and gastrointestinal problems — and in animal studies, Wegovy increased the risk of thyroid cancer. But many people frustrated with not being able to lose weight solely through diet and exercise were prepared to put up with these risks to finally see an impact on their size and their health.
While many clinicians presumed the drugs would improve heart health because obesity is a major risk factor for heart disease, Novo Nordisk invested in its largest ever trial — following over 17,000 people since 2018 — to obtain proof.
The initial result was “out of this world”, says Martin Lange, executive vice-president of development for Novo Nordisk. The data from trials suggests the drug is not just having an impact by helping people lose weight, he says, but also by lowering blood pressure, changing the balance of lipids in the blood, and reducing inflammation.
He adds that this significant impact on people who already have heart disease suggests that the drug may also help cut the risk for people who have not yet developed it.
The full study, which will be released at an academic conference and is yet to be peer reviewed, will also show the drug’s impact on 28 other factors, including on other expensive conditions such as kidney disease.
Analysts believe the trial could also help build confidence that the benefits of the drug outweigh the risks, after the European regulator began investigating reports of patients having suicidal thoughts, and a US lawsuit claiming that Novo and Lilly’s diabetes drugs cause “stomach paralysis”. Lange says the heart trial showed no causal link with suicidal ideation or thyroid cancer. Novo and Lilly have said patient safety is their top priority.
Novo hopes that being armed with this data will help it overcome the competition when Lilly enters the obesity market with its own medication, which is in the same category of so-called GLP-1 receptor agonists.
But Lilly believes Novo’s data will also help it sell its weight loss drug, even though the results of its comparable trial won’t be ready until 2027. On an earnings call this week, Lilly’s president of diabetes, Mike Mason, declared it a “fantastic day for people living with obesity”.
“It is an important milestone in a long-term goal to get broad access for anti-obesity medications,” he said.
The question of coverage
Despite Wegovy’s sudden ubiquity in popular culture, less than half of all US commercial insurers cover the weight loss drug. Medicare, the government-backed insurance for seniors, is prohibited by Congress from paying for any obesity medications.
In Europe, where supply problems mean Novo Nordisk is only just slowly starting to launch the drug, public health systems are restricting who qualifies for it. The UK’s National Institute for Health and Care Excellence (Nice), for example, will pay for it for patients at the higher end of the body mass index, who already have at least one weight-related condition, and then for only two years, despite evidence showing that if you stop taking the drug, you regain weight.
After the heart trial data, financial analysts believe this reluctance will have to change. BMO’s Seigerman says not covering a drug that is potentially life-saving will be “unethical”.
“Before the data, a lot of these weight loss drugs were seen as vanity, no one was treating obesity as a medical condition, a precursor to negative health outcomes,” he explains. “If you have cancer, they are not going to deny you a cancer drug. Now, you’re going to treat obesity as a medical condition.”
Novo Nordisk’s Jørgensen says that with broader coverage, the company hopes to reach more patients with the highest BMIs and comorbidities, even though “some of the less fortunate individuals will not be able to pay out of pocket”.

Novo and other drugmakers have been lobbying Congress to pass a law reversing the ban on Medicare coverage of drugs designed to address obesity. Seigerman is optimistic that the new data will eventually help pass the law, because it will prove that it will save Medicare money in the long run, on hospitalisations for heart attacks and strokes.
Stacie Dusetzina, professor of health policy at Vanderbilt University, says it is “incredibly difficult” for legislation to pass through Congress at the moment. But she said the fact that Medicare does cover semaglutide for treatment of diabetes suggests that, after the new data, it could cover the drug for people with cardiac conditions. The Centers for Medicare & Medicaid Services did not respond to a request for comment.
In the UK, Jørgensen says Nice could be willing to expand coverage when a trial shows the health benefits go beyond two years.
Trained as a health economist, he says the drugs could help not just save healthcare costs but also get people back to work. “If you can turn them back from being consumers of healthcare services to consume less of those and actually be active in the workforce and taxpayers, that economic model would be very beneficial for most typical European societies where healthcare is funded by tax payments,” he says.
‘Very hard to justify’
But for insurers and health systems, the conundrum has barely changed.
David Rind, chief medical officer at the Institute for Clinical and Economic Review, a non-profit that estimates fair prices for the US health system, says he does not think the heart trial has changed the ethics of not covering the drugs.
ICER’s cost effectiveness assessment concluded they represented low long-term value for money — and it already assumed the obesity medications had some cardiac benefit.
The biggest problem is the sheer number of people who could qualify for the drug: at current prices, ICER estimated that only 0.1 per cent could be treated within five years without “major budget disruptions” for the insurers.
“The options are to move money away from other healthcare, raise premiums, or taxes if you’re the government, or manufacturers could lower the price to a cost effective price and still make enormous amounts of money because enormous numbers of patients want this,” Rind says.
Drugmakers will be reluctant to offer big discounts on such a popular drug, but the opacity of the US drug pricing system means they could do deals to offer larger rebates if volumes increase.
Gelfand, who represents large US employers that provide health benefits, says the price tag is still “very hard to justify”, particularly given patients have to take the drug for life. He expects employers to stick to covering very narrow patient groups, if any at all.
Otherwise, he says, premiums in the US could rise 15 to 20 per cent, increasing the likelihood that healthy people stop buying insurance, and making the remaining population riskier to cover.
A single heart trial was never likely to change payers’ position on the drugs overnight, and even if it had, Novo Nordisk would not have enough of the drug to supply the world.
But it could be the first in a series of data releases that proves how weight loss drugs prevent the serious consequences of obesity — eventually reshaping not just patients, but also the healthcare industry and the broader economy.
Emily Field, an analyst at Barclays, says that the benefits of the drugs are “only just starting to be understood” and eventually, doctors will become used to treating obesity before other health problems develop.
“This is like the smartphone, it is going to change society in a big way,” she says. “This trial is a huge validation that it is not just cosmetic.”
Additional reporting by Jamie Smyth in New York
This story originally appeared on: Financial Times - Author:Hannah Kuchler




























