US criticised for rolling out Covid boosters without human trials
Health experts have warned that the US decision to roll out new coronavirus boosters without clinical testing on humans risks denting public trust and increasing hesitancy about vaccines.
The Joe Biden administration is using the bivalent boosters, which contain the original Covid-19 strain and the genetic code of the Omicron sub-variants BA.4 and BA.5, to vaccinate more Americans against the virus.
It has bought 171mn doses of the BioNTech/Pfizer and Moderna boosters for $5bn and fast-tracked their authorisation before human trials are complete, hoping that they provide better protection against the dominant variants than existing Covid vaccines.
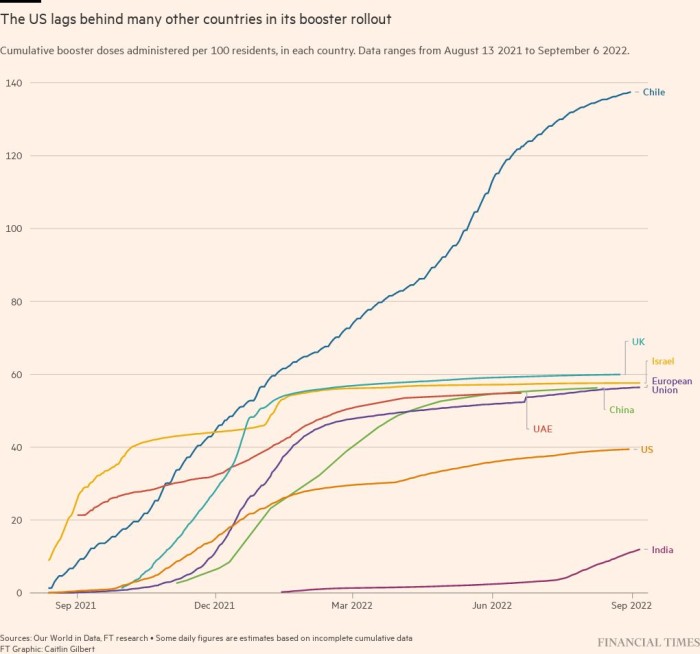
Last month, the UK became the first country to approve a bivalent booster. But it waited for clinical data to greenlight a shot targeting the original Covid strain and the BA.1 sub-variant, which has since been superseded by BA.4/BA.5.
On Monday, Pfizer and BioNTech said the European Medicines Agency had recommended the BA.4/BA.5 bivalent booster for conditional marketing authorisation based on data from the companies’ bivalent jab targeting BA.1 and pre-clinical data.
Some scientists agree, noting that new clinical data are not always required when the influenza jab is tweaked annually to target the latest strains.
Nancy Jecker, professor at University of Washington School of Medicine, said the risk of approving the boosters was minimal given they are not significantly different to the original Covid jabs.
“Emergency authorising Moderna and Pfizer boosters based on animal studies gives people the chance to minimise their risk of Covid as more people congregate indoors during fall and winter months,” said Jecker.
But Topol and Offit argued the US should have waited for evidence the boosters are better than the existing jabs.
“I don’t think they have proven that this influenza vaccine strategy — where we pick influenza strains every March for a vaccine that comes out in September — makes sense for Covid,” said Offit.
He said there is no evidence showing the BA.4/BA.5 booster provided better protection than existing jabs. Initial clinical data for the BA.1 bivalent booster showed it produced virus-fighting antibody levels that were 1.5 to 1.75 times higher than the existing jab, which was not a clinically significant difference, Offit said.
The CDC cites polling which suggests 72 per cent of respondents would definitely or probably receive bivalent boosters. If uptake of the jabs reach similar levels to the annual flu vaccine — half of US adults — by early autumn CDC modelling predicts it would prevent 100,000 hospitalisations, save 9,000 lives and billions of dollars in healthcare costs.
To boost uptake the CDC has simplified its guidance on eligibility, recommending a single bivalent booster for everyone over 12 years of age who has already received a Covid vaccine.
But workers on the frontline of the inoculation drive are sceptical the public will rush to get another jab.
“I think demand will continue to be sporadic because those people who want to be vaccinated have already got the vaccine and been boosted,” said Rene Rodriguez, owner of Tens Pharmacy in Roseland, a small town in New Jersey.
“There are really two types of people: those who believe in the vaccine, who are generally up to date with their jabs, and those that don’t.”
This story originally appeared on: Financial Times - Author:Caitlin Gilbert


























